Gregg Keaney and co-workers at H3 Biomedicine have reported in Org Lett on the synthesis of 6-deoxypladienolide D. OL paper
OL paper
Month: October 2014
Dr. Crasto’s news on Continuous flow for drug synthesis
As I mentioned in the past week, I will take some time to provide some of Anthony Melvin Crasto’s information of flow approaches to drug discovery and API synthesis — maybe I should rename this CF list of the week – we shall see. It certainly opened my eyes to how much work is going on in pushing flow chemistry to application in drug discovery — and so many of our readers want to keep up with the latest thoughts and trends. Here is this week’s news:
A report (Org Process Res Dev 2014, ASAP article) out of Jamison’s group at MIT, provides a 3-step synthesis of Rufinamide in 92% overall yield. The process illustrates a continuous and convergent method, moving away from the isolation of a key organic azide intermediates and a Cu coiled-tube reactor for the cycloaddition reaction to the corresponding desired triazole.
View original post 222 more words
Microwave to Continuous Flow Tech Transfer
I’m not sure if it was in the generic scheme of the reactions performed, the authors of the work or the title translating microwave methods to flow that got me: truth is it was a bit of all three in a recent publication (Beilstein JOC 2011). The scheme had a para-nitro diaryl ether, which for me means an amine handle with multiple substitution patterns on both rings — reliving my sorafenib days at Bayer ( I did a lot of Ullmann reactions, uugh, although the microwave did prove handy!). The authors of course are front and center of the flow chemistry community and lastly there have been a number of papers taking microwave methods and transferring them into flow methods — and I do believe we will see more of these. It’s meaningful and there are advantages to both.
Although Dr. Watts and Dr. Wiles probably didn’t have…
View original post 772 more words
Comparison between microwave to flow technology – tech transfer
Although I found an example in the literature in moving from a microwave method to a micro-reactor method – and placed it in my synthflow resource blog, it should also be placed here — there are advantages born out in each technology depending on the application. To give yourself something to think about, and there are going to be several more publications using this theme, follow the paper described.
Sex hormones, Adrenal cortisol, Prostaglandins
Leaders in Pharmaceutical Business Intelligence (LPBI) Group
Sex Hormones, Adrenal Cortisol, Prostaglandins
Curator: Larry H. Bernstein, MD, FCAP
Steroids
A major class of lipids, steroids, have a ring structure of three cyclohexanes and one
cyclopentane in a fused ring system as shown below. There are a variety of functional
groups that may be attached. The main feature, as in all lipids, is the large number of
carbon-hydrogens which make steroids non-polar.
Steroids include such well known compounds as cholesterol, sex hormones, birth
control pills, cortisone, and anabolic steroids.
http://www.elmhurst.edu/~chm/vchembook/images/556cholesterol.gif
The best known and most abundant steroid in the body is cholesterol. Cholesterol is
formed in brain tissue, nerve tissue, and the blood stream. It is the major compound
found in gallstones and bile salts. Cholesterol also contributes to the formation of
deposits on the inner walls of blood vessels. This topic was covered in the previous
discussion of the lipids series, and extensively in cardiovascular topics.
Cholesterol is synthesized by the liver from carbohydrates and proteins as well as fat.
Therefore, the elimination of cholesterol rich foods from the diet does not necessarily
lower blood cholesterol levels. Some studies have found that if certain unsaturated fats
and oils are substituted for saturated fats, the blood cholesterol level decreases.
The research is incomplete on this problem.
Cholesterol exists as an ester with fatty acids.What is the functional group at carbon 3
which is used to make an ester?
OH is alcohol
What is the feature on carbon 17?
Branched long hydrocarbon chain
Sex Hormones
http://www.elmhurst.edu/~chm/vchembook/images/556sexhormones.gif
The primary sex hormones, testosterone and estrogen, are responsible for the
development of secondary sex characteristics. Two female sex hormones,
progesterone and estrogen or estradiol control the ovulation cycle. Notice
that the male and female hormones have only slight differences in structures,
but yet have very different physiological effects.
Testosterone promotes the normal development of male genital organs and
is synthesized from cholesterol in the testes. It also promotes secondary male
sexual characteristics such as deep voice, facial and body hair.
Estrogen, along with progesterone regulates changes occurring in the uterus
and ovaries known as the menstrual cycle. Estrogen is synthesized from
testosterone by making the first ring aromatic which results in the loss of a
methyl group and formation of an alcohol group.
List three functional groups in progesterone?
C#3 & #17 are ketones; C#4&5 are alkenes;
What is difference between progesterone and testosterone?
testosterone has C#17 alcohol vs ketone on progesterone
What is difference between testosterone and estrogen?
Estrogen has C#3 alcohol, + aromatic first ring;
no methyl group on C#17
Adrenocorticoid Hormones
The adrenocorticoid hormones are products of the adrenal glands.
The most important mineralcorticoid is aldosterone, which regulates the
reabsorption of sodium and chloride ions in the kidney tubules and increases
the loss of potassium ions.Aldosterone is secreted when blood sodium ion
levels are too low to cause the kidney to retain sodium ions. If sodium
levels are elevated, aldosterone is not secreted, so that some sodium
will be lost in the urine. Aldosterone also controls swelling in the tissues.
Cortisol, the most important glucocortinoid, has the function of increasing
glucose and glycogen concentrations in the body. These reactions are
completed in the liver by taking fatty acids from lipid storage cells and
amino acids from body proteins to make glucose and glycogen.
In addition, cortisol is elevated in the circulation with cytokine mediated
(IL1, IL1, TNFα) inflammatory reaction, called the systemic inflammatory
response syndrome. Its ketone derivative, cortisone, has the ability
to relieve inflammatory effects. Cortisone or similar synthetic derivatives
such as prednisolone are used to treat inflammatory diseases, rheumatoid
arthritis, and bronchial asthma. There are many side effects with the use
of cortisone drugs, such as bone resorption, so there use must be
monitored carefully. Cortisol is increased pathologically with the growth
of a pituitary gland tumor that secretes adrenocorticotropic hormone
(ACTH), called Addison’s Disease, which is also associated with
hirsuit features.
What is the only difference between cortisol and aldosterone?
Aldosterone has C#13 aldehyde instead of methyl group
http://www.elmhurst.edu/~chm/vchembook/images/556cortisone.gif
Prostaglandins
Prostaglandins, are like hormones in that they act as chemical messengers,
but do not move to other sites, but work right within the cells where
they are synthesized. (PARACRINE)
Prostaglandins are unsaturated carboxylic acids, consisting of of a 20 carbon
skeleton that also contains a five member ring. They are biochemically
synthesized from the fatty acid, arachidonic acid.
http://www.elmhurst.edu/~chm/vchembook/images/551arachidonic.gif
The unique shape of the arachidonic acid caused by a series of cis double
bonds helps to put it into position to make the five member ring.
Prostaglandins are unsaturated carboxylic acids, consisting of a
- 20 carbon skeleton that also contains
- a five member ring and
- are based upon the fatty acid, arachidonic acid.
There are a variety of structures one, two, or three double bonds. On the
five member ring there may also be double bonds, a ketone, or alcohol groups.
In PGE2, list all of the functional groups.
one acid; two alkenes; two alcohols; one ketone
What is difference the C=C double bonds?
the upper is cis; the lower is trans.
http://www.elmhurst.edu/~chm/vchembook/images/556prostaglandin.gif
Functions of Prostaglandins
There are a variety of physiological effects including:
- Activation of the inflammatory response, production of pain, and fever.
When tissues are damaged, white blood cells flood to the site to
try to minimize tissue destruction. Prostaglandins are produced
as a result. - Blood clots form when a blood vessel is damaged. A type of
prostaglandin called thromboxane stimulates constriction and
clotting of platelets. Conversely, PGI2, is produced to have the
opposite effect on the walls of blood vessels where clots
should not be forming. - Certain prostaglandins are involved with the induction of labor
and other reproductive processes. PGE2 causes uterine
contractions and has been used to induce labor. - Prostaglandins are involved in several other organs such as
the gastrointestinal tract (inhibit acid synthesis and increase
secretion of protective mucus), increase blood flow in kidneys,
and leukotriens promote constriction of bronchi associated
with asthma.
When you see that prostaglandins induce inflammation, pain, and fever,
what comes to mind but aspirin. Aspirin blocks an enzyme called
cyclooxygenase, COX-1 and COX-2, which is involved with the ring
closure and addition of oxygen to arachidonic acid converting to
prostaglandins.
The acetyl group on aspirin is hydrolzed and then bonded to the
alcohol group of serine as an ester. This has the effect of blocking
the channel in the enzyme and arachidonic can not enter the active
site of the enzyme.
By inhibiting or blocking this enzyme, the synthesis of prostaglandins
is blocked, which in turn relives some of the effects of pain and fever.
http://www.elmhurst.edu/~chm/vchembook/images/556coxaspirin.gif
http://www.elmhurst.edu/~chm/vchembook/
Sphingolipids
View original post 39 more words
FLOW SYNTHESIS IN ACTION
I.R. Baxendale, S.C. Schou, J. Sedelmeier, S.V. Ley, Chem. Eur. J. 2010, 16, 89-94.
READ
http://onlinelibrary.wiley.com/doi/10.1002/chem.200902906/abstract
Multi-step in flow: The palladium-catalysed acylation of terminal alkynes for the synthesis of yne![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) ones as well as their further transformation to various heterocycles in a continuous-flow mode is presented. Furthermore, an extension of the simple flow configuration that allows for easy batch splitting and the generation of a heterocyclic library is described (see scheme).
ones as well as their further transformation to various heterocycles in a continuous-flow mode is presented. Furthermore, an extension of the simple flow configuration that allows for easy batch splitting and the generation of a heterocyclic library is described (see scheme).
Piecing together the puzzle: understanding a mild, metal free reduction method for large scale synthesis of hydrazines
D.L. Browne,* I.R. Baxendale, S.V. Ley, Tetrahedron 2011, 67, 10296-10303.
http://www.sciencedirect.com/science/article/pii/S0040402011015304

A key intermediate for the synthesis of hydrazines via a mild, metal free reduction of diazonium salts has been isolated and characterized by X-ray analysis. The presence of this intermediate is general, as demonstrated by the preparation of a number of analogues. A discussion of the mechanism and potential benefits of such a process are also described.
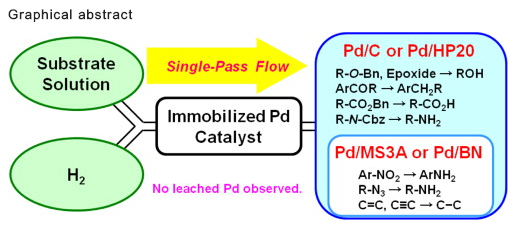
Evaluation of Pd-catalyzed hydrogenations under flow conditions
Just finished reading an excellent example from a group in Japan moving a batch hydrogenation to flow. Dr Sajiki’s group developed and evaluated a number of Pd-catalysts and their effectiveness in an H-Cube (Thales Nano) reactor. This evaluation went into detail on both reaction optimization and chemoselectivities, depending on the catalyst type — while scoping a variety of temp, flow and solvents for each of the reactions — it was truly impressive how much work was performed and the amount of information we obtain for the work done. The work was published in Tetrahedron 2014 and featured and a highlight brief in Synfacts 2014.
From the cartoon below, you can see that the substrate was passed through a series of immobilized Pd catalysts to provide different targets depending on their functionality. A variety of pressures, flow rates, and substrate concentrations were studied as part of the optimization process.
Rather than…
View original post 66 more words
PRISMANE 棱晶烷

650-42-0 cas
Tetracyclo[2.2.0.02,6.03,5]hexane


| Prismane | |||
|---|---|---|---|
|
|||
 |
|||
| Identifiers | |||
| CAS number | 650-42-0 |
||
| ChemSpider | 16736515 |
||
| Jmol-3D images | Image 1 | ||
| Properties | |||
| Molecular formula | C6H6 | ||
| Molar mass | 78.11 g mol−1 | ||

History
In the mid 19th century, investigators proposed several possible structures for benzene which were consistent with its empirical formula, C6H6, which had been determined by combustion analysis. The first, which was proposed by Kekulé in 1867, later proved to be closest to the true structure of benzene. This structure inspired several others to propose structures that were consistent with benzene’s empirical formula; for example, Ladenburg proposed prismane, Dewar proposed Dewar benzene, and Koerner and Claus proposedClaus’ benzene. Some of these structures would be synthesized in the following years. Prismane, like the other proposed structures for benzene, is still often cited in the literature, because it is part of the historical struggle toward understanding the mesomeric structures and resonance of benzene. Some computational chemists still research the differences between the possible isomers of C6H6.[3]
Properties
Prismane is a colourless liquid at room temperature. The deviation of the carbon-carbon bond angle from 109° to 60° in a triangle leads to a high ring strain, reminiscent of that of cyclopropane but greater. The compound is explosive, which is unusual for a hydrocarbon. Due to this ring strain, the bonds have a low bond energy and break at a low activation energy, which makes synthesis of the molecule difficult; Woodward and Hoffmann noted that prismane’s thermal rearrangement to benzene is symmetry-forbidden, comparing it to “an angry tiger unable to break out of a paper cage.”[4]
The substituted derivative hexamethylprismane (in which all six hydrogens are substituted by methyl groups) has a higher stability, and was synthesized by rearrangement reactionsin 1966.[5]
Synthesis
The synthesis starts from benzvalene (1) and 4-phenyltriazolidone, which is a strong dienophile. The reaction is a stepwise Diels-Alder like reaction, forming a carbocation as intermediate. The adduct (2) is then hydrolyzed under basic conditions and afterwards transformed into a copper(II) chloride derivative with acidic copper(II) chloride. Neutralized with a strong base, the azo compound (3) could be crystallized with 65% yield. The last step is a photolysis of the azo compound. This photolysis leads to a biradical which forms prismane (4) and nitrogen with a yield of less than 10%. The compound was isolated by preparative gas chromatography.

Et2O
-45 °C, 45 %


+

Et2O, Dioxane
0 °C to RT, 60 min, 50-60 %


MeOH, H2O
Reflux, 24 h


H2O
65 % (2 steps)


PhMe
30 °C, 5 h, 8 %


| References |
|---|

https://www.beilstein-journals.org/bjoc/single/printArticle.htm?publicId=1860-5397-7-30

http://chemistry.stackexchange.com/questions/8898/does-benzene-have-isomers-and-resonance-structures
References
- Ladenburg A. (1869). “Bemerkungen zur aromatischen Theorie“. Chemische Berichte 2: 140–2. doi:10.1002/cber.18690020171.
- Katz T. J., Acton N. (1973). “Synthesis of Prismane”. Journal of the American Chemical Society 95 (8): 2738–2739. doi:10.1021/ja00789a084.
- UD Priyakumar, TC Dinadayalane, GN Sastry (2002). “A computational study of the valence isomers of benzene and their group V hetero analogs”. New J. Chem. 26 (3): 347–353.doi:10.1039/b109067d.
- R. B. Woodward and R. Hoffmann, Angew. Chem., Int. Ed. Engl., 8, 789, (1969)
- Lemal D. M., Lokensgard J. P. (1966). “Hexamethylprismane”. Journal of the American Chemical Society 88 (24): pp 5934–5935. doi:10.1021/ja00976a046.
Microwave Labs: Florida State University – Gregory Dudley
Professor Dudley’s recent work in microwave chemistry has certainly heated up the place. His group is trying to dive into more complex studies surrounding microwave acceleration above the normal predicted amount. I have enjoyed the pursuit of his work over the last couple of years and we should see continued efforts coming out soon. Take a look at some of his recent contributions at Florida State — seems the university has a number of efforts in microwave methodologies to keep track.