Masahiro Terada and co-workers from Tohoku University have reported in Organic Letters on a copper-catalyzed rearrangement to form alpha,beta-unsaturated ketonitrones.
Month: July 2014
Peganumine A
Hui-Ming Hua and co-workers from Shenyang Pharmaceutical University have reported in Organic Letters on the isolation of peganumine A, which had an IC50 value of 5.8 μM against HL-60 cells.
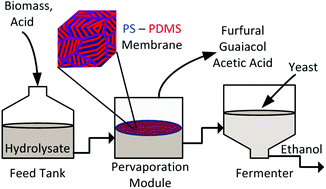
Fermentation of hydrolysate detoxified by pervaporation through block copolymer membranes
Fermentation of hydrolysate detoxified by pervaporation through block copolymer membranes
The large-scale use of lignocellulosic hydrolysate as a fermentation broth has been impeded due to its high concentration of organic inhibitors to fermentation. In this study, pervaporation with polystyrene-block-polydimethylsiloxane-block-polystyrene (SDS) block copolymer membranes was shown to be an effective method for separating volatile inhibitors from dilute acid pretreated hydrolysate, thus detoxifying hydrolysate for subsequent fermentation. We report the separation of inhibitors from hydrolysate thermodynamically and quantitatively by detailing their concentrations in the hydrolysate before and after detoxification by pervaporation. Specifically, we report >99% removal of furfural and 27% removal of acetic acid with this method. Additionally, we quantitatively report that the membrane is selective for organic inhibitor compounds over water, despite water’s smaller molecular size. Because its inhibitors were removed but its sugars left intact, pervaporation-detoxified hydrolysate was suitable for fermentation. In our fermentation experiments, Saccharomyces cerevisiae strain SA-1 consumed the glucose in pervaporation-detoxified hydrolysate, producing ethanol. In contrast, under the same conditions, a control hydrolysate was unsuitable for fermentation; no ethanol was produced and no glucose was consumed. This work demonstrates progress toward economical lignocellulosic hydrolysate fermentation.
E-mail: nbalsara@berkeley.edu ;
Tel: +1 (510) 642-8937
E-mail: aparkin@lbl.gov ;
Tel: +1 (510) 643-5678
DOI: 10.1039/C4GC00756E
Received 28 Apr 2014, Accepted 24 Jun 2014
First published online 11 Jul 2014
Hydrolysate was pervaporated with a block copolymer membrane, removing inhibitors but leaving sugars, creating a viable fermentation broth.
Rhodium cascade
Richmond Sarpong and co-workers have reported in ACIE on a rhodium catalyzed cascade reaction involving an aza-Cope rearrangement.
Anthony crasto’s blog New drug approvals touches 3 lakh views…….Helping millions

link is http://newdrugapprovals.org/
All about Drugs, live, by DR ANTHONY MELVIN CRASTO, Worlddrugtracker, Helping millions, 7 million hits on google, pushing boundaries, one lakh plus connections worldwide, 3 lakh plus VIEWS on this blog in 193 countries

THANKS AND REGARD’S
DR ANTHONY MELVIN CRASTO Ph.D
web link

New Drug Approvals, ALL ABOUT DRUGS, WORLD DRUG TRACKER
MEDICINAL CHEM INTERNATIONAL, DRUG SYN INTERNATIONAL
SCALEUP OF DRUGS, ALL FOR DRUGS ON WEB,
MY CHINA, VIETNAM AND JAPAN BLOGS
ICELAND, RUSSIA, ARAB
BOBRDOBR, BLAND ICELAND, 100zakladok, adfty
GROUPS
you can post articles and will be administered by me on the google group which is very popular across the world
OPD GROUPSPACES, SCOOP OCI, organic-process-development GOOGLE, TVINX, MENDELEY WDT, SCIPEOPLE OPD,EPERNICUS OPD, SYNTHETIC ORGANIC CHEMISTRYLinkedIn group, DIIGO OPD, LINKEDIN OPD, WDT LINKEDIN, WDTI ZING

![]()
Cyanogramide
Weiming Zhu and co-workers at Ocean University of China have reported in Organic Letters on the isolation of cyanogramide.
Indole alkaloid synthesis
Corey Stephenson and colleague at the University of Michigan have reported in JACS on the synthesis of pseudotabersonine. A mental roadblock for me was when I was looking at the paper, I was trying to find the structure of catalyst 5, which was the iridium catalyst used for a ring opening reaction, but just found the number 5 in the scheme. Within the text of the paper however it says Ir(dF(CF3)ppy)2(dtbbpy)PF6, which was cited with a Sigma Aldrich catalog number.
Crinipellin A synthesis
Hee-Yoon Lee and co-workers at KAIST have reported in JACS on the synthesis of crinipellin A.
Tetrapetalone A-Me glycon synthesis
Alison Frontier and co-workers at Rochester University and Hoveyda Boston College have reported in ACIE on the synthesis of tetrapetalone A-Me glycon.

Chemists succeed in isolating carbon-gold compound of “amazing stability”

Gold carbene model: The Au=C double bond in the gold carbene compound is the bond between the large golden atom in the middle and the slightly greenish atom below. The position of the atoms was derived from an x-ray crystal structure analysis. Credit: Matthias Hussong and Bernd F. Straub, Heidelberg University
With a chemical “trick”, scientists at Heidelberg University have succeeded in isolating a stable gold carbene complex. Chemist Prof. Dr. Bernd F. Straub and his team are the first to have created the basis for directly examining the otherwise unstable gold-carbon double bond. Prof. Straub explains that highly reactive gold carbene molecules play an important role in landmark catalysing processes taking place at high speed. The research findings have been published in the German and the international edition ofAngewandte Chemie, a journal on applied and fundamental chemistry.
Chemical reactions can be accelerated with the aid of catalysts…
View original post 345 more words