DRUG REGULATORY AFFAIRS INTERNATIONAL

Arylboronic acids, but not the corresponding deboronated arenes, recently have been found to be weakly mutagenic in microbial assays [1]. Hence arylboronic acids may be considered potentially genotoxic impurities, and controlling the levels of residual arylboronic acids in APIs could become a regulatory requirement. The issues should be decided by toxicology studies for the specific arylboronic acids in question.
Several approaches have been successful in removing boronic acids. Diethanolaminomethyl polystyrene (DEAM-PS) [2],[3] and immobilized catechol [4] have been used to scavenge boronic acids. Complex formation with diethanolamine may solubilize residual boronic acids in mother liquors. Since arylboronic acids ionize similarly to phenols, basic washes of an API solution may remove arylboronic acids. A selective crystallization can purge an arylboronic acid from the API.
The best means to control residual aryl boronic acids in APIs at the ppm level may be to decompose them through deboronation. Sterically hindered, electron-rich aryl boronates…
View original post 3,373 more words


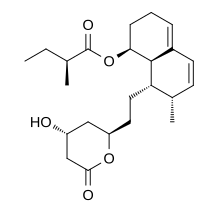 Mevastatin (compactin, ML-236B) is a
Mevastatin (compactin, ML-236B) is a 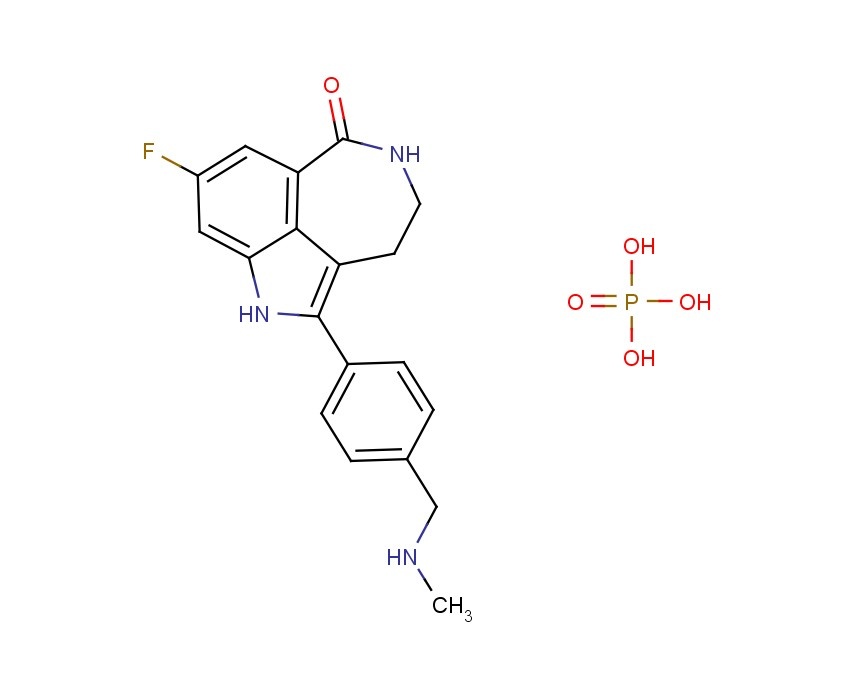 AG014699,
AG014699, 






 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO …..

 LIONEL MY SON
LIONEL MY SON
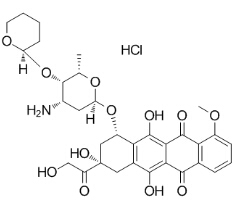 Pirarubicin Hydrochloride
Pirarubicin Hydrochloride 

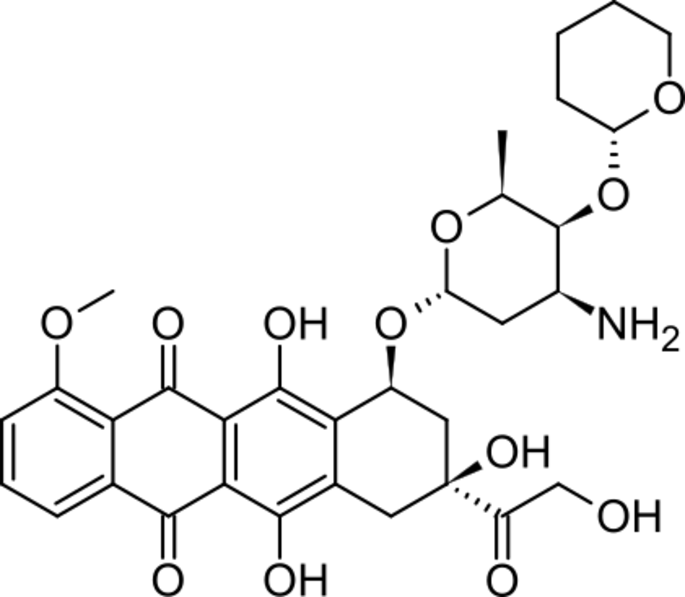
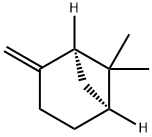

 .
. .
.
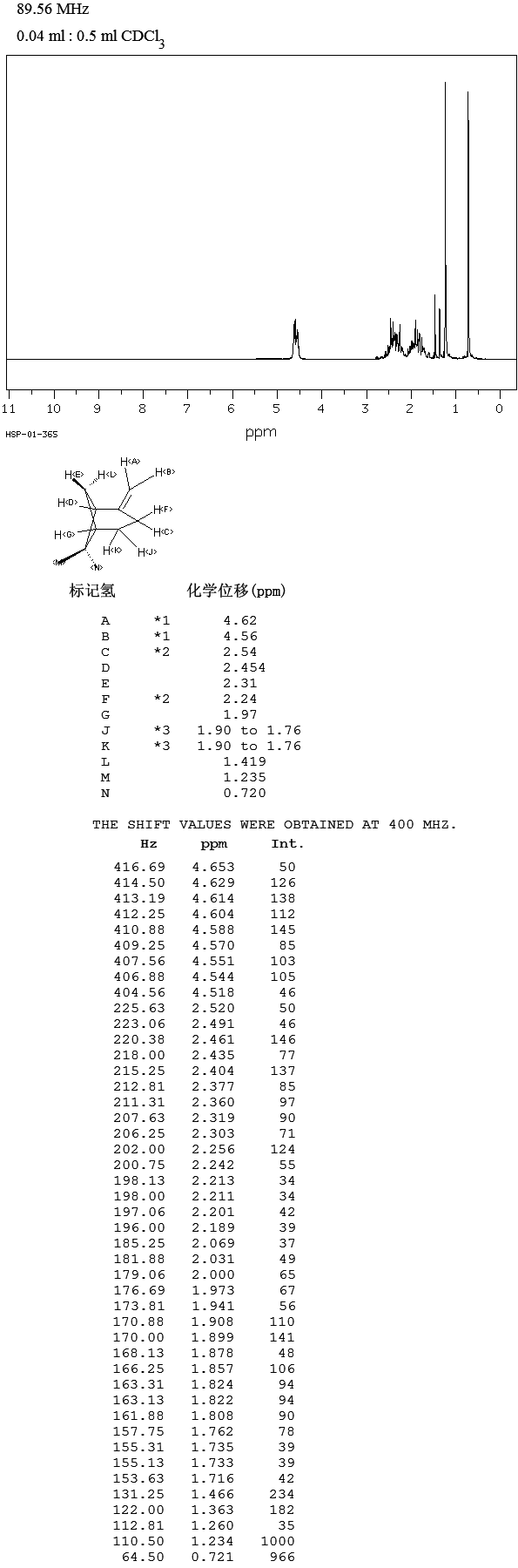
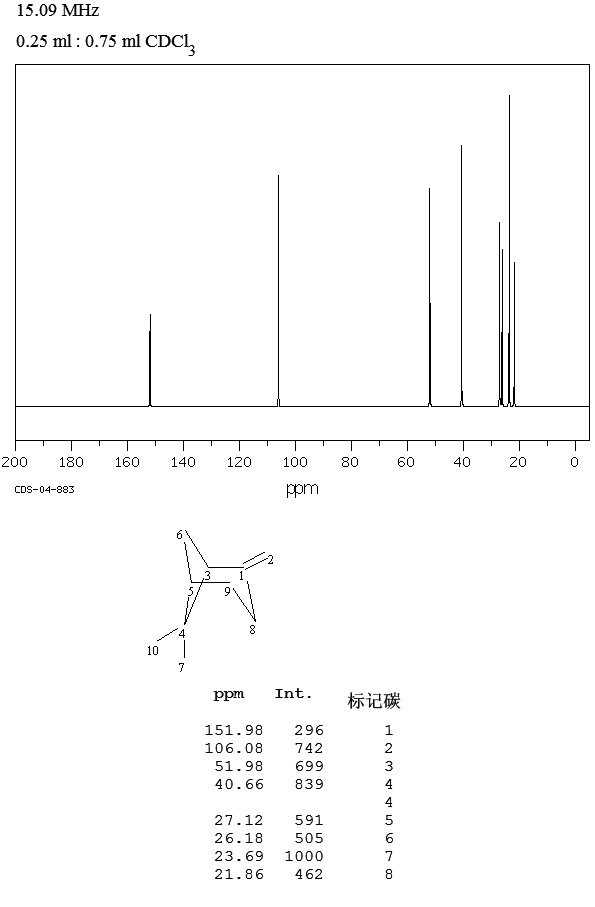 .
. .
. .
. .
. .
.
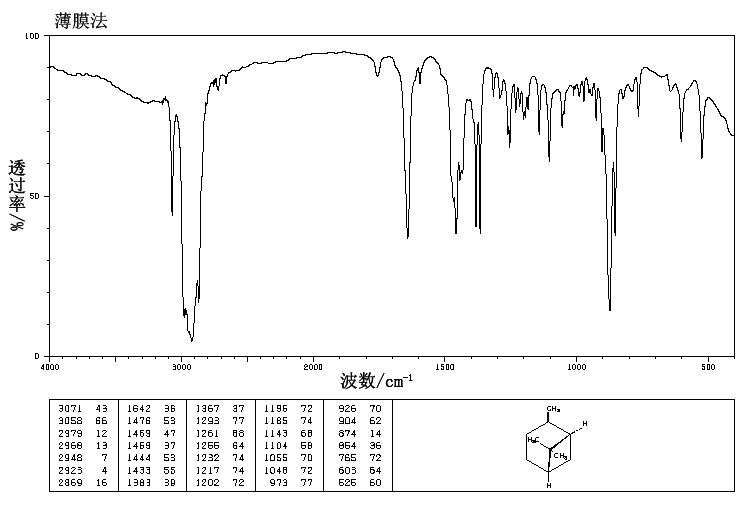 .
. .
.