
A new synthetic route to hainanolidol and harringtonolide offers flexibility to access other members of Cephalotaxus norditerpenes


A new synthetic route to hainanolidol and harringtonolide offers flexibility to access other members of Cephalotaxus norditerpenes

Benzannulation of phthalic anhydrides with alkynes to polyfunctionalized naphthalenes and phenanthrenes was confirmed to be straightforward using a palladium catalytic system. Sequential liberation of CO2 and CO occurred via oxidative decomposition of anhydride. In the case of 1,8-naphthalenedicarboxylic anhydrides, both aryls were encompassed in the annulation reaction to afford acenaphthylenes.
allow slideshare to load………….
New Drug Discovery from natural products
In the present article, we describe SAR studies within a series of N-hydroxy-dihydronaphthyridinone HIV integrase inhibitors that led to a candidate compound, PF-4776548, of high potency and with an excellent resistance profile. Uncertainties around the human pharmacokinetic predictions for PF-4776548 led to the compound being taken into a human microdose study to confirm its human pharmacokinetics, the results of which are described herein.
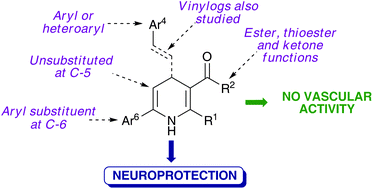
A library of 4,6-diaryl-1,4-dihydropyridines was synthesized using a CAN-catalyzed, Hantzsch-related three component reaction starting from ammonium acetate, β-dicarbonyl compounds and a variety of α,β-unsaturated ketones including chalcones, their vinylogs and heteroanalogues. These compounds lack the structural features needed for vascular activity and were found to prevent calcium overload and behave as neuroprotective agents. One of the compounds, bearing a 2-thienyl substituent at C-4, showed the highest neuroprotective activity and was also a moderate antioxidant, being a good lead compound for further studies in this area.

There are a lot of chemicals racing around your brain and body when you’re in love. Researchers are gradually learning more and more about the roles they play both when we are falling in love and when we’re in long-term relationships. Of course, estrogen and testosterone play a role in the sex drive area . Without them, we might never venture into the “real love” arena.
That initial giddiness that comes when we’re first falling in love includes a racing heart, flushed skin and sweaty palms. Researchers say this is due to the dopamine, norepinephrine and phenylethylamine we’re releasing. Dopamine is thought to be the “pleasure chemical,” producing a feeling of bliss. Norepinephrine is similar to adrenaline and produces the racing heart and excitement. According to Helen Fisher, anthropologist and well-known love researcher from Rutgers University, together these two chemicals produce elation, intense energy, sleeplessness, craving, loss of appetite and focused attention. She also says, “The human body releases the cocktail of love rapture only when certain conditions are met and … men more readily produce it than women, because of their more visual nature.”
Researchers are using functional magnetic resonance imaging (fMRI) to watch people’s brains when they look at a photograph of their object of affection. According to Helen Fisher, a well-known love researcher and an anthropologist at Rutgers University, what they see in those scans during that “crazed, can’t-think-of-anything-but stage of romance” — the attraction stage — is the biological drive to focus on one person. The scans showed increased blood flow in areas of the brain with high concentrations of receptors for dopamine — associated with states of euphoria, craving and addiction. High levels of dopamine are also associated with norepinephrine, which heightens attention, short-term memory, hyperactivity, sleeplessness and goal-oriented behavior. In other words, couples in this stage of love focus intently on the relationship and often on little else.
Another possible explanation for the intense focus and idealizing view that occurs in the attraction stage comes from researchers at University College London. They discovered that people in love have lower levels of serotonin and also that neural circuits associated with the way we assess others are suppressed. These lower serotonin levels are the same as those found in people with obsessive-compulsive disorders, possibly explaining why those in love “obsess” about their partner.

Oxetanes as Versatile Elements in Drug Discovery and Synthesis
Johannes A. Burkhard, Georg Wuitschik, Mark Rogers-Evans, Klaus Müller and Erick M. Carreira