Month: April 2013
Benign by design: catalyst-free in-water, on-water green chemical methodologies in organic synthesis
Benign by design: catalyst-free in-water, on-water green chemical methodologies in organic synthesis
http://pubs.rsc.org/en/content/articlelanding/2013/cs/c3cs60025d
Received 25 Jan 2013, First published online 26 Mar 2013
Catalyst-free reactions developed during the last decade and the latest developments in this emerging field are summarized with a focus on catalyst-free reactions in-water and on-water.
Various named reactions, multi-component reactions and the synthesis of heterocyclic compounds are discussed including the use of various energy input systems such as microwave- and ultrasound irradiation, among others.
Organic chemists and the practitioners of this art both in academia and industry hopefully will continue to design benign methodologies for organic synthesis in aqueous media under catalyst-free conditions by using alternative energy inputs based on fundamental principles.
Compounds Discovered Entirely by Mistake
Whether the product of poor lab housekeeping, clumsy researchers, or an inexplicable urge to taste everything, there are some discoveries that would never have been made on purpose.
1. Artificial Sweeteners
Until the introduction of neotame in 2002, every common artificial sweetener had been discovered by mistake, usually by a chemist accidentally ingesting one of their compounds [1]. This trend started with saccharin in the 19th century, when Constantin Fahlbert was working in the lab of Ira Remsen. After a long day of creating new toluene derivatives, Fahlberg left for dinner without washing his hands. Breaking open a dinner roll, he found the bread to be unnaturally sweet, and eventually traced the cause back to a chemical residue left on his hands. Together with Remsen he purified the residue and together they published a paper titled, “On the Oxidation of ortho-Toluenesulfonimide” (in German of course)…
View original post 1,085 more words
Discovery of MK-1220 (Hepatitis C NS3/4A protease inhibitor)
A recent letter in ACS Med.Chem.Lett. details the optimisation of a series of inhibitors of the Hepatitis C virus (HCV) NS3/4A protease, and is interesting in that it provides detailed pharmacokinetic data for a series of macrocyclic lactones, a class of therapeutic agents with physicochemical properties that fall outside the typical range with which most medicinal chemists are familiar.
The current front line therapy for HCV infection (which can lead to liver cirrhosis) involves treatment with PEGylated interferon α (PEGasys or PEG-Intron, by injection) and ribavirin (orally). Unfortunately this approach has limited efficacy due, in part, to the low completion rates observed for the lengthy (~1 year) course of therapy. The clear need for improved therapies and the associated commercial opportunity has led to many companies progressing molecules into the clinic (see this overview of the area), a number of which have targeted NS3/4A protease. The NS3/4A protease represents…
View original post 460 more words
Gila monster lizard’s poison helps diabetics
This video from the USA is called The Gila Monster – AMAZING Venomous Lizard Encounter!
From The Herald in Britain:
Lizard’s poison may be boon for diabetics
It is one of only two venomous lizards in the world, but the Gila Monster could become an unlikely boon for the health of tens of thousands of Scots.
The poison of the large reptile, native to Mexico and the southern United States, has been found to contain a chemical similar to a human hormone that helps regulate the blood sugar level of diabetics.
Two feet long, with a diet comprising small mammals and birds, the lizard is an unlikely source of medicine.
This month, a new type 2 diabetes drug, exenatide, based on the chemical from the garish pink and black lizard, Heloderma suspectum, is available in the UK.
In Scotland, around 150,000 people suffer type 2 diabetes while a further…
View original post 107 more words
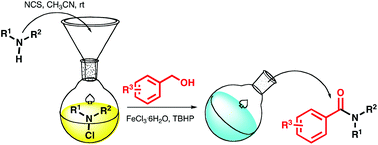
Iron-catalysed oxidative amidation of alcohols with amines
Iron-catalysed oxidative amidation of alcohols with amines
DOI: 10.1039/C3OB40170G, Paper
General procedures for amides 4a-q:
An amine (0.32 mmol) was added to a solution of N-chlorosuccinimide (0.352 mmol) in 10 mL of acetonitrile under N2 atmosphere and at room temperature. The reaction was monitored by TLC until disappearance of the
amine (1-2 hours), then were added an alcohol (1.6 mmol), TBHP (1.6 mmol, 0.22 mL of a 70 wt% in water) and FeCl3.6H2O (0.045 mmol) under N2 atmosphere.The resulting reaction mixture was heated in an oil bath at 85°C (the reaction was monitored by TLC until disappearance of Nchloroamine). Then the reaction mixture was quenched with 20 mL of a saturated solution of Na2SO3 (for removal of excess TBHP)
and extracted three times with 40 mL of diethyl ether. The combined organic phases were dried over anhydrous Na2SO4 and the solvent was evaporated under reduced pressure. The crude product was purified by silica gel column chromatography to provide the desired amides 4a-q
Inverse electron demand Diels-Alder (iEDDA)-initiated conjugation: a (high) potential click chemistry scheme
-
Inverse electron demand Diels-Alder (iEDDA)-initiated conjugation: a (high) potential click chemistry scheme
Chem. Soc. Rev., 2013, Advance Article
DOI: 10.1039/C3CS60049A, Tutorial ReviewAstrid-Caroline Knall, Christian SlugovcGraz, AustriaInverse electron demand Diels-Alder reactions (iEDDA) between 1,2,4,5-tetrazines and olefins have emerged as a versatile conjugation tool in the sense of “click chemistry” and are therefore the subject of this tutorial review.Inverse electron demand Diels–Alder reactions (iEDDA) between 1,2,4,5-tetrazines and olefins have emerged into a state-of-the art concept for the conjugation of biomolecules. Now, this reaction is also increasingly being applied in polymer science and materials science. The orthogonality of this exciting reaction to other well-established click chemistry schemes, its high reaction speed and its biocompatibility are key features of iEDDA making it a powerful alternative to existing ligation chemistries. The intention of this tutorial review is to introduce the reader to the fundamentals of inverse electron demand Diels–Alder additions and to answer the question whether iEDDA chemistry is living up to the criteria for a “click” reaction and can serve as a basis for future applications in post-synthetic modification of materials
Rotamers- assigned by a simple NMR experiment
Rotamers are conformational isomers where interconversion by rotation around a single bond is restricted and an energy barrier has to be overcome in order to convert one conformer to another. When this rotation strain barrier is high enough to allow for the isolation of the conformers then the isomers become atropoisomers. Rotamers are however not separable and their existence normally complicates the 1H NMR interpretation. Variable temperature (VT) NMR is the generally preferred method for studying the equilibration of the rotamers and at low temperatures the spectrum is assigned to the frozen equilibrium and multiple peaks are observed while at higher temperatures the spectrum simplifies as the equivalent peaks are averaged out. Other methods for NMR simplification include the introduction of a complexing agent and solvent switching. All these techniques are inconvenient to the organic synthetic chemist when working on small scale. To overcome this problem, Steve Ley and…
View original post 332 more words
Paper published online in Organic Letters
Our latest paper was published online in Organic Letttersas an ‘as soon as possible’ article. The paper describes the use of trifluoromethyl-substituted vinylsulfonium salts for the synthesis of saturated heterocycles containing the CF3 group. The epoxide-fused heterocycles are expected to be of interest to medicinal chemists in particular. This work was carried out in collaboration with Prof Varinder Aggarwal, Sven Fritz and Tom West at the University of Bristol. We thank EPSRC and SFI for funding.
 The paper can be read (with a subscription) at the following link:
The paper can be read (with a subscription) at the following link:
Organic Letters 2012, asap; DOI: 10.1021/ol303200n
Don’t have a subscription? email Eoghan for an electronic preprint:
(eoghan_dot_mcgarrigle_at_ucd_dot_ie).
Eugenol 1H NMR Interpretation
 EUGENOL EUGENOL |
|
| CAS:97-53-0 | |
 |
|

Eugenol is a phenylpropene, an allyl chain-substituted guaiacol. Eugenol is a member of the phenylpropanoids class of chemical compounds. It is a clear to pale yellow oily liquid extracted from certain essential oils especially from clove oil, nutmeg, cinnamon, basil andbay leaf.It is slightly soluble in water and soluble in organic solvents. It has a spicy, clove-like aroma.